Eblasakimab is a novel biologic in development for moderate-to-severe AD, with the potential to deliver a monthly dosing regimen from initiation with no compromise on efficacy.
About eblasakimab
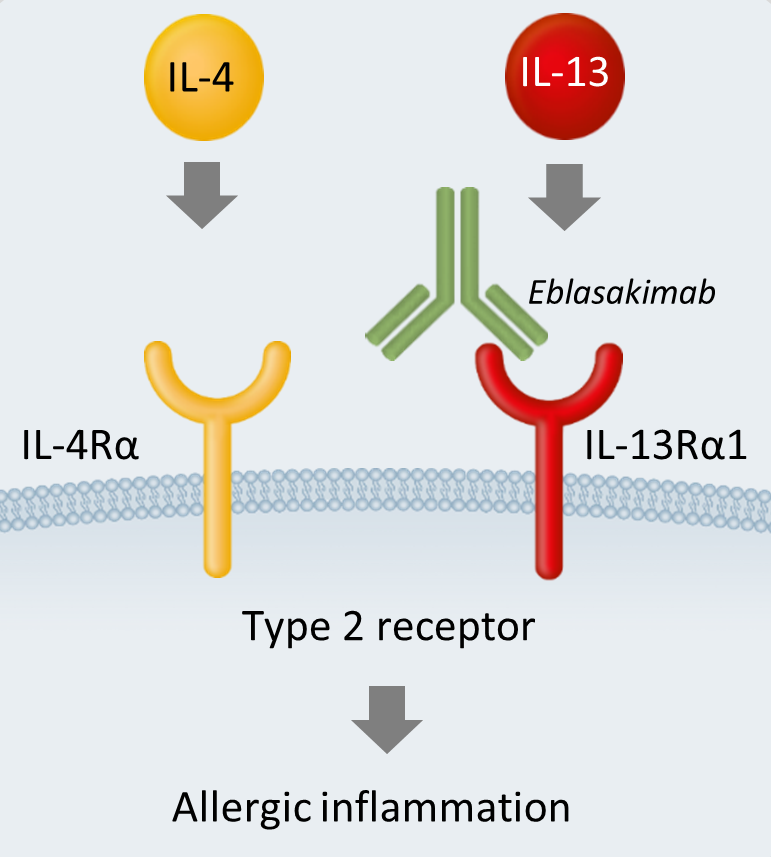
Eblasakimab is a novel, fully human, monoclonal IgG4 antibody that binds specifically to the Interleukin (IL)-13 receptor (IL-13R), preventing signaling of IL-4 and IL-13 through the Type 2 receptor.
The role of the Type 2 receptor in driving allergic conditions
The Type 2 receptor is a heterodimer comprising of the IL-4 receptor (IL-4R) and the IL-13 receptor (IL-13R). Cytokines IL-4 and IL-13 signal through the Type 2 receptor and are key drivers of inflammation, central to triggering symptoms of allergy in Type 2-driven diseases such as AD, asthma and COPD.
Eblasakimab's mechanism of action in AD
Eblasakimab is the only known antibody targeting the IL-13 receptor in clinical development for the treatment of moderate-to-severe AD.
By targeting the IL-13 receptor, eblasakimab's novel approach selectively blocks the Type 2 receptor, preventing signaling through both IL-4 and IL-13.
Watch the following video to learn more about eblasakimab's mechanism of action in AD:
What makes eblasakimab different?
Eblasakimab and dupilumab both block Type 2 receptor signaling, but recent translational data demonstrated that eblasakimab’s blocking of IL-13R resulted in lower levels of allergy related Th2 cytokines and pro-inflammatory Th1 cytokines compared to blockade of the IL-4R, the target of dupilumab. Hence eblasakimab could lead to more efficient blockade of the Type 2 receptor compared to dupilumab.
Additionally, unlike dupilumab, by sparing the Type 1 receptor, eblasakimab may offer a differentiated therapeutic approach with a better safety profile.
Eblasakimab may be effective against other allergic co-morbidities, unlike drugs targeting the IL-13 ligand
Drugs targeting the IL-13 ligand, like lebrikizumab and tralokinumab, do not inhibit IL-4 signaling through the Type 2 receptor, which also contributes to inflammation in AD and other allergic conditions.
78% of AD patients have Type 2 inflammatory comorbidities[1] like asthma and allergic rhinitis. By blocking both IL-4 and IL-13 signaling through the Type 2 receptor, eblasakimab has the potential to be efficacious in a broad range of allergic co morbidities in AD, unlike drugs targeting the IL-13 ligand.
A proven molecular basis for directly attenuating itch
In our published data, blocking IL-13R with eblasakimab in human sensory neurons was shown to significantly reduce cytokine-driven amplification of itch and spontaneous activation of neurons.
Itch is a key burdensome symptom in patients with AD and these results suggest a molecular basis for the significant reduction of pruritus scores observed in clinical studies of eblasakimab.
Posters on translational studies presented at international scientific conferences can be viewed on our publications page.
You can also view data from our previous studies on eblasakimab by clicking the links below.
Ongoing studies
TREK-DX
Phase 2 study in dupilumab experienced moderate-to-severe AD patients
TRials in EblasaKimab- Dupilumab eXperienced (TREK-DX) is a double-blind, randomized, placebo controlled, Phase 2 study of eblasakimab in adults with moderate-to-severe atopic dermatitis who have previously been treated with dupilumab. The primary endpoint in the study is percentage change in Eczema Area Severity Index (EASI) score from baseline to week 16. For more information about the trial, follow this link.
[1] Calzavara-Pinton et al (2023) Adv Ther 40:5366-5382