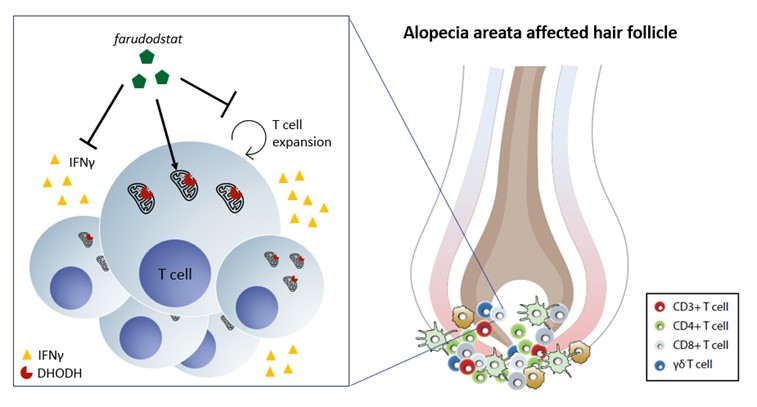
Farudodstat is an orally active, potent inhibitor of the enzyme dihydroorotate dehydrogenase (DHODH), that suppresses T cell proliferation and secretion of Interferon Gamma (IFNγ) by blocking de novo production of pyrimidines required for DNA replication.
DHODH inhibitors
The inhibition of DHODH is an established mechanism for the treatment of several autoimmune diseases. DHODH inhibitors have been approved and widely used for Multiple Sclerosis and Rheumatoid Arthritis, however first-generation inhibitors, such as teriflunomide, have limited potency and safety liabilities. In contrast, farudodstat is structurally distinct from and up to two orders of magnitude more potent at inhibiting DHODH and in limiting T cell activity, and has a well-tolerated safety profile.
Clinical development in Alopecia Areata
Farudodstat is currently in clinical development for alopecia areata (AA). AA is an autoimmune disease characterized by partial or complete hair loss on the scalp and body. AA is associated with severe psychological burden and has limited treatment options, affecting over 700,000 patients in the US.
The pathophysiology of AA is driven by the loss of Immune Privilege (IP) in the hair follicles as measured by an increase in expression of MHC I protein. IP loss is accompanied by the proliferation of interferon gamma (IFNγ)-secreting immune cells, which attack the hair follicles and lead to subsequent hair loss.
Click here to read more about alopecia areata.
Mechanism of action in AA
AA is believed to result from a loss of immune privilege in the hair follicle following a triggering event (eg stress, infection or trauma), mediated by IFNγ. In vitro studies have shown that farudodstat inhibits T cell proliferation and IFNγ production. Recent translational studies in an ex vivo human AA disease model showed that farudodstat reduced T cell proliferation, expression of MHC proteins and the number of MHC II+ cells in the hair bulb, suggesting potential protection from IP loss. The model also yielded other signs of efficacy suggesting that farudodstat may potentially protect against hair follicle attack by activated T cells.
Based on this translational data and the safety profile of farudodstat characterized in clinical studies to date, we believe farudodstat has the potential to be a first-in-class therapy addressing the unmet needs in AA.
Ongoing studies
FAST-AA
Phase 2 proof-of-concept trial in AA patients initiated in the second quarter of 2023
FArudodstat STudy in Alopecia Areata” (FAST-AA) is a Phase 2a proof-of-concept trial evaluating the efficacy and safety of the study drug, farudodstat in participants with at least 30% scalp hair loss due to AA.
Participants will be randomized to receive either farudodstat or placebo, orally, twice daily for 12 weeks. Following this initial study treatment, participants will crossover to the other arm for an additional 12 weeks of study treatment, followed by a 4-week safety follow-up. The study is expected to enroll approximately 60 adults across at least 20 sites in the US. The primary endpoint will utilize the Severity of Alopecia Tool (SALT) for clinical assessment and evaluate percentage change in SALT score from baseline to week 12. Key secondary endpoints to be studied include the proportion of participants achieving a SALT score ≤ 20 and the proportion of participants achieving SALT50 and SALT75 at week 12. For more information about the trial, click here.